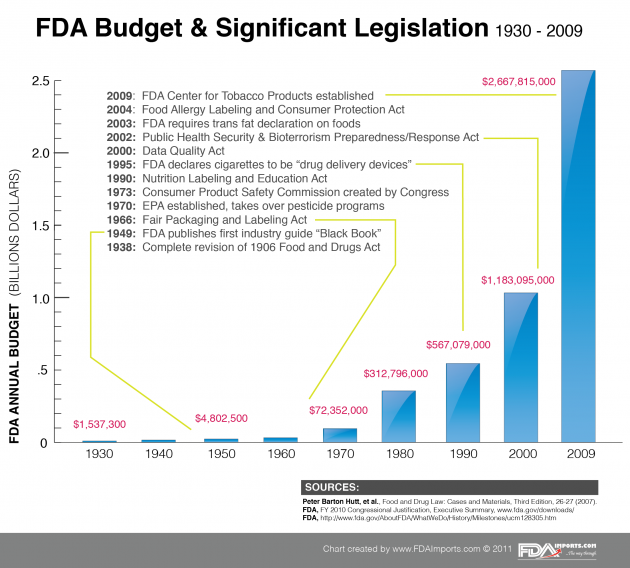
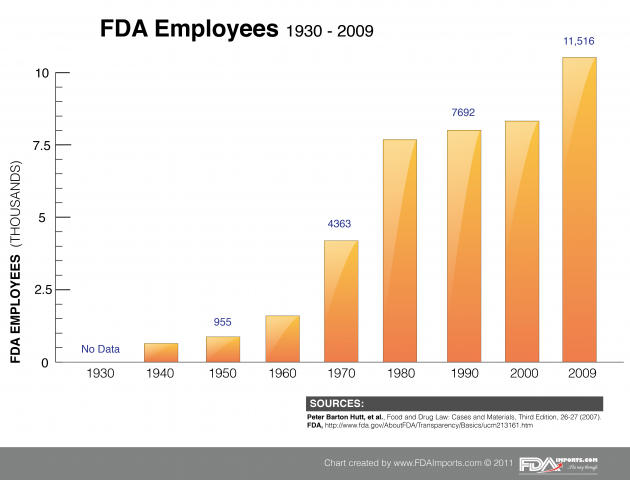
The Food and Drug Administration is no small government agency. In 2009 it boasted a 2.6 billion dollar budget and employed over 11,500 people spread from its suburban Maryland Headquarters to various national and international offices. The charts and data below illustrate how the ever-expanding global network of importing and exporting has increased the roles (and burden) of FDA in regard to regulatory oversight. As globalization continues to impact specific industries and the speed of communication and transport increases, we can only expect to see increased spending, staffing and legislation from FDA in the years ahead. The FDAImports.com team brings decades of regulatory experience together to assist our clients with navigating complex FDA regulation.
Significant Rulings, Legislation and Events in FDA History
1933: Complete revision of 1906 Food and Drugs Act
1949: FDA publishes first industry guide “Black Book”
1966: Fair Packaging and Labeling Act
1970: EPA established, takes over pesticide programs
1973: Consumer Product Safety Commission created by Congress
1990: Nutrition Labeling and Education Act
1995: FDA declares cigarettes to be “drug delivery devices”
2000: Data Quality Act
2002: Public Health Security & Bioterrorism Preparedness/Response Act
2003: FDA requires trans fat declaration on foods
2004: Food Allergy Labeling and Consumer Protection Act
2009: FDA Center for Tobacco Products established