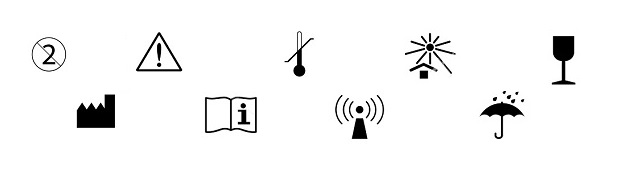
The Food and Drug Administration (FDA) issued the final rule for using symbols in labeling of medical devices and certain biological products.[1] The rule will be effective mid-September 2016. Specifically, the new regulations allow symbols to “stand alone”, i.e., without adjacent explanatory text, under certain conditions. This goes a long way to harmonize with foreign government requirements, including the European Union (EU). Medical devices labeled with “stand alone” symbols may be imported into the US provided that they meet the FDA conditions below.
Manufacturers may continue to use symbols accompanied by explanatory text. However, this seems to defeat the purpose of using a symbol, and becomes cumbersome because the text must be in all of the languages used where the device will be marketed. Therefore, we expect the industry will resist adding the written verbiage, and will rely on Agency guidance for using the stand-alone images wherever possible.
Under the final rule, manufacturers can use “stand-alone” symbols under one of the following conditions:
- The symbol is included in a standard recognized by FDA and is used according to the specifications in the standard.
- The symbol is included in a standard developed by a standards development organization (even if not recognized by FDA), and is used according to the specifications set forth in the standard.
- The symbol is in a standard recognized by FDA but is not used according to the standard.
If the manufacturer wants to use a symbol under options 2 or 3, they must determine “that the symbol is likely to be read and understood by the ordinary individual under customary conditions of purchase and use.”[2]
Under any of these conditions, the symbol must be included in a symbols glossary included in the device labeling, and the labeling must bear a prominent, conspicuous statement identifying the location of the symbols glossary, which may be printed in the labeling or made available electronically at the manufacturer’s website. The symbols glossary is a compiled listing of:
- Each SDO-established symbol used in the labeling for the device;
- The title and designation number of the SDO-developed standard containing the symbol;
- The title of the symbol and its reference number, if any, in the standard; and
- The meaning or explanatory text for the symbol as provided in the FDA recognition.
In a separate Code of Federal Regulations (CFR) notice, FDA updated its list of recognized standards to include applicable device symbol standards. FDA defines an SDO as a nationally or internationally recognized organization that follows a process open to public scrutiny; where participation is balanced; where an appeals process is included; where the standard is not in conflict with any FDA statute, regulation, or policy; and where the standard is national or international in scope.
The final rule also confirms the use of the symbol “Rx only” in place of the statement “Caution: Federal law restricts this device to sale by or on the order of a [licensed practitioner].”
Devices diverted from the EU to the US
The issue of importing devices that were originally labeled for foreign markets containing stand-alone symbols would appear to be largely resolved. However, the importer must be cognizant of two issues. First, the importer must either ensure that the symbols are specified in an FDA-recognized standard or determine that “the symbol is likely to be read and understood by the ordinary individual.” Second, unless one is provided by the manufacturer, importers will likely have to provide an FDA-formatted symbols glossary, which is not specified in foreign declarations or standards.
Importers or manufacturers with questions about how this new rule applies to their existing products, or who need to apply the rule to new product labeling, can contact FDAImports.com for support. With over 100 years of former-FDA experience on our staff, we focus on getting medical devices into the US market.

