Recent headline: Trump to Pharma CEOs: 75% to 80% of FDA Regulations Will be Eliminated Our take... Yeah...not so fast. I expect the Trump-team follow up to be, "we meant 75-80% of the really bad regulations will disappear -- and most likely only in...
Law & Regulatory
Powdered Medical Gloves Bite the Dust
FDA essentially banned all powdered medical gloves in the United States starting on Wednesday, January 18, 2017. On that date, not only are the gloves no longer allowed to be manufactured or distributed, but also healthcare providers must dispose of their stocks too....
Discussion Points: FDA’s New Deeming Regulations for Tobacco Products
On May 5, 2016, FDA finalized its tobacco deeming regulations, which will extend its authority to all tobacco products, including e-cigarettes, cigars, hookah tobacco, and pipe tobacco products. These deeming regulations are intended to help implement the Family...
There’s Always a Price to Pay – Food Industry Members Take Note
Since FY 2015, Congress has continuously raised the issue of inadequate border screening of food. Particularly, Congress has been concerned with designing an import control strategy that incorporates a comprehensive system of safeguard reviews, rather than solely...
Now, Back to our Regularly Scheduled Program: FSMA Compliance Dates Remain Largely Unchanged
UPDATE 07-07-2017: The next compliance date is in March 2018. Importers should spend this time developing the programs as it takes time to work with Foreign Suppliers to build a robust FSVP program. FDA recently published a Federal Register notice announcing delays...

General Wellness: Policy on Low Risk Devices
If you manufacture wearable fitness trackers or electronic massagers, determining how your product will be regulated is an important exercise. An erroneous conclusion can have a substantial impact on your business. At the end of July 2016, FDA published a final...

FSVP: Requirements for Hazard Analysis, Risk Evaluation, and Supplier Compliance
UPDATE 07-07-2017: The next compliance date is in March 2018. Importers should spend this time developing the programs as it takes time to work with Foreign Suppliers to build a robust FSVP program. Importers, get ready: in May 2017, FDA will start enforcing its final...
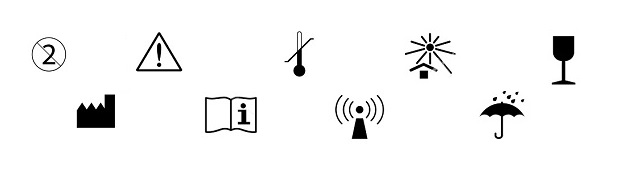
Breakthrough in the Use of Symbols in Medical Device Labeling
The Food and Drug Administration (FDA) issued the final rule for using symbols in labeling of medical devices and certain biological products.[1] The rule will be effective mid-September 2016. Specifically, the new regulations allow symbols to “stand alone”, i.e.,...
Congress’ New Move on Catfish Inspection and the Implications
Recently, Congress made a new move to nullify the USDA rule published in December 2015 to establish a catfish inspection program under USDA's Food Safety and Inspection Service (FSIS). On May 25, 2016, the Senate passed a joint resolution to disapprove USDA’s...
What Will the Updated Nutrition Facts Panel Mean for the Food Industry?
UPDATE 6/5/2018: Editor’s Note: On May 4, 2018, FDA published a rule stating it will be extending the compliance dates for the Nutrition Facts panel and Serving Size Final Rules to January 1, 2020 for manufacturers with $10 million or more in annual food sales....

