Can you spell “ex-am-in-ation”?
Even if you can spell it, can you define it? If you can’t then you won’t have any surprises this fall while you’re importing food to the USA. If you can correctly define what an “examination” is then you’d better sit down before you continue reading this.
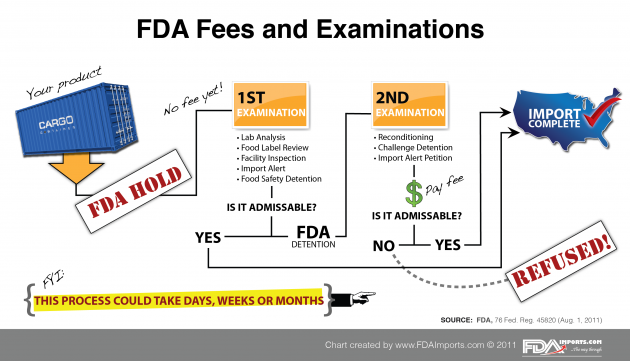
The U.S. Food and Drug Administration (FDA) examines only 2% of the shipments entering the U.S. market under its jurisdiction, including imported foods. Starting October 1, 2011, under the Food Safety Modernization Act, FDA will beginning charging food importers a fee (based upon an hourly rate) for any “re-examination” that is required because FDA discovered a violation related to food safety during the initial examination.
Now, common sense dictates how this should work: (a) FDA samples imported shrimp and tests it, finding it contaminated with Salmonella, (b) FDA detains the shipment due to a violation that happens to relate to food safety, (c) the importer requests permission to cook (recondition) the shrimp to remove the food-safety-related violation, (d) FDA permits the reconditioning and re-examines the shipment by evaluating the proposed cooking method, supervising the sampling or cooking process, or inspecting the shipment prior to release. That sounds like a re-examination of imported food due to a violation related to food safety. So, FDA will charge a fee for the steps that relate to the re-examination.
Enter Uncommon Sense
FSMA requires FDA to conduct two examinations of imported food before FDA is authorized to charge a fee for services related to the second and subsequent examinations. Unfortunately for importers, FDA has so broadly defined what constitutes the “first examination” that virtually every imported food shipment detained for an apparent food safety reason will become subject to the new FDA tax (oops) … re-examination fee. U.S. food importers, most of them small businesses, will be charged $224 per hour (plus expenses) for any such re-examinations. Those expenses will be passed on to consumers resulting in a dramatic increase to food costs in the USA.
In its August 1, 2011 Federal Register notice, FDA stated that a first examination occurs whenever it reviews information to determine an imported food’s admissibility and that review results in an FDA detention related to a food safety problem. FDA’s “first examination” definition includes tasks and operations that clearly do not involve any FDA inspection of the imported food at all, such as reviewing sample results from a third party, reviewing any relevant epidemiological evidence, reviewing a third party facility inspection, and almost any activity related to an FDA import alert. This is jaw-dropping. FDA’s own examples admit that no FDA examination of the imported food is occurring – yet FDA calls these activities the “first examination!
After FDA’s initial “examination,” the importer must pay FDA for any other review FDA conducts on the same food shipment. And FDA charges $224 per hour! Talk about overhead! Any FDA activity related to future shipments of food (e.g., through FDA’s import alert program) are considered re-examinations. FDA lists four examples of when the importer can expect to pay a fee.
1. When a food is reconditioned or relabeled to address a food safety concern.
2. When an importer is seeking release of an imported food that FDA has detained – even for FDA automatic detentions.
3. When an importer or foreign food manufacturer petitions FDA requesting removal from FDA import alert.
4. When FDA supervises destruction of FDA refused cargo.
There are 2 major problems here (See chart).
This conduct should be offensive to anyone who thinks the US Government should be working within the confines of the law granting authority to regulate. FDA could not convince Congress to give the agency a food importation user fee so FDA made one up out of the re-examination fee language of FSMA.
This conduct is outrageous because it runs contrary to 100 years of FDA policy, procedure and statutory authority. Since 1906 FDA has had the authority to detain and refuse admission to imported food if it “appears from the examination of samples or otherwise” that the imported food violates the law. So engrained is this system that FDA called detentions that happen under the “or otherwise” language “automatic detentions”. After getting sued (and losing) four times, FDA finally stopped using the “automatic detention” language and converted to “Detention without physical examination” – get that? “Without” examination.
Obviously reviewing third party inspection reports, third party test results, reviewing epidemiology reports, and detentions without examination cannot be an “examination” of any kind. But FDA counts all of those activities as “examinations” so anything FDA does after those “examinations” related to imported food safety will result in a tax (oops…again) … fee.
Got fees?
Although there’s a slim chance that these changes are motivated by higher regulatory ideals, FDA appears to be looking at this fee requirement as a simple means to capture additional operational funds through a user fee system rejected by Congress in other food safety bills. It’s not the first time we’ve seen this happen in history. “Every time a coin in coffer rings, another shipment from re-examination springs.” Regardless of motive, FDA’s interpretation will dramatically increase the costs imposed on food importers and secondarily, to consumers.
No one can afford this FDA. No one.