That Catfish and its Nine Lives
No one with any experience or regulatory memory at the Food and Drug Administration (FDA) or the United States Department of Agriculture (USDA) wanted the regulation of catfish (and its many cousins) transferred from FDA to USDA. USDA's regulatory requirements are...
Let the Latex Go: How FDA’s Leak Test Sampling Method can Hold Back Compliant Shipments
When it comes to leak testing for latex products (condoms, medical gloves), FDA is not infallible, and can be challenged. When testing for leak holes in shipments of imported condoms and medical gloves, the Agency adopts a composite sampling method, which increases...
Hanjin Updates: Fall 2016
September 22, 2016: Korean Air’s Board (Hanjin’s sister company in the same conglomerate group) has authorized a $60 million transfusion to facilitate Hanjin vessels’ cargo unloading in the U.S. ports. Below are quick updates regarding upcoming schedules. Hanjin...
There’s Always a Price to Pay – Food Industry Members Take Note
Since FY 2015, Congress has continuously raised the issue of inadequate border screening of food. Particularly, Congress has been concerned with designing an import control strategy that incorporates a comprehensive system of safeguard reviews, rather than solely...
Now, Back to our Regularly Scheduled Program: FSMA Compliance Dates Remain Largely Unchanged
UPDATE 07-07-2017: The next compliance date is in March 2018. Importers should spend this time developing the programs as it takes time to work with Foreign Suppliers to build a robust FSVP program. FDA recently published a Federal Register notice announcing delays...

General Wellness: Policy on Low Risk Devices
If you manufacture wearable fitness trackers or electronic massagers, determining how your product will be regulated is an important exercise. An erroneous conclusion can have a substantial impact on your business. At the end of July 2016, FDA published a final...

FSVP: Requirements for Hazard Analysis, Risk Evaluation, and Supplier Compliance
UPDATE 07-07-2017: The next compliance date is in March 2018. Importers should spend this time developing the programs as it takes time to work with Foreign Suppliers to build a robust FSVP program. Importers, get ready: in May 2017, FDA will start enforcing its final...
Conclusion on FDA, Swordfish, and Methyl Mercury
In April 2015, we commented on how FDA cancelled Import Alert 16-08 (Detention without Physical Examination of Swordfish for Methyl Mercury). This alert instructed FDA to automatically detain all swordfish imports unless the manufacturer was on FDA’s Green List....
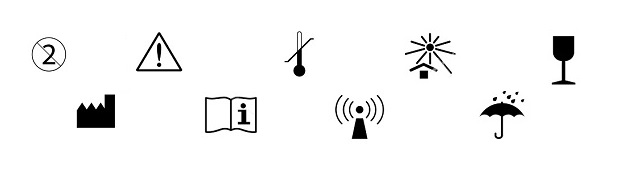
Breakthrough in the Use of Symbols in Medical Device Labeling
The Food and Drug Administration (FDA) issued the final rule for using symbols in labeling of medical devices and certain biological products.[1] The rule will be effective mid-September 2016. Specifically, the new regulations allow symbols to “stand alone”, i.e.,...
Congress’ New Move on Catfish Inspection and the Implications
Recently, Congress made a new move to nullify the USDA rule published in December 2015 to establish a catfish inspection program under USDA's Food Safety and Inspection Service (FSIS). On May 25, 2016, the Senate passed a joint resolution to disapprove USDA’s...
