Honey and Sweetener: Coexisting No Longer
Last month, FDA issued a new Draft Guidance, requiring the labels of honey products to declare sweeteners in the statement of identity. This sounds ridiculous. Consumers have accepted that honey marketers routinely mix honey and sweeteners – for example, sugar or corn...
More than E-Cigs: FDA’s Proposed Rule about Extending Jurisdiction to All Tobacco Products
The news media is flooded with stories about FDA proposing to regulate electronic cigarettes (e-cigs) for the first time, according to the proposed rule published on April 24. Despite the news stories and FDA’s talking points, the proposed rule talks very little...
Boston Seafood Show 2014: Presentation and Discussion
FDAImports.com and ExportToUsa.com.cn attended the Boston Seafood Show this year on Mar.16-18. The team took the opportunity to speak with seafood producers from around the world, who think 2014 will be a harvest year. US importers at the show expressed the desire...
USDA and Catfish: Do They Really Want It?
U.S. Department of Agriculture (USDA) officials testified before Congress on April 10, 2014 that the Department responsible for protecting agricultural interests in the U.S.A. will not meet the 60-day deadline to issue a proposed rule governing its new authority to...
Lawsuit Against FDA Gets Seafood Exporter Onto Green List
The team at FDAImports.com successfully persuaded FDA to return an exporter to the Green List for Import Alert 16-131 (Detention Without Physical Examination of Aquacultured Catfish, Basa, Shrimp, Dace, and Eel from China- Presence of New Animal Drugs and/or Unsafe...
USDA’s New Jurisdiction over Catfish, Pangasius, Swai, Tra, and Basa
On February 7, 2014, the U.S. federal government enacted the Agricultural Act of 2014 (the “Farm Bill”). This bill gives USDA exclusive authority to regulate and inspect all farm-raised catfish sold in the U.S. In doing so, it removes FDA’s regulatory control over...
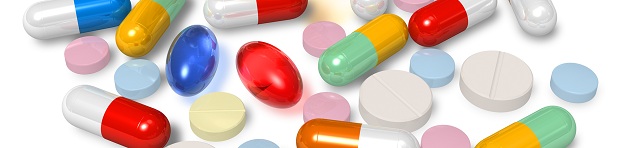
The Five W’s of Adverse Event Reporting for OTC Drugs
The Dietary Supplement and Nonprescription Drug Consumer Protection Act amended the FFDCA to subject those OTC drugs marketed without an approved NDA or ANDA to OTC drug reporting requirements, which had previously only applied to those OTC drugs with an approved...
FDAImports.com at the Boston Seafood Show 2014
FDA has recently begun applying Import Alert 99-30 to breaded seafood from China. That import alert relates to melamine in dairy products and foods with dairy-based ingredients. This is an unprecedented action. FDA has previously only applied Import Alert 99-30 to...
Import Alert 99-30: Now For Seafood Too?
The seafood industry should start preparing for greater FDA scrutiny and interference with seafood products from China. The industry is quite familiar with FDA’s Import Alert 16-131, which requires testing all aquaculture from China for unapproved veterinary drugs...
cGMP Violations: Don’t Learn it the Hard Way
Intending to close the frequency gap between foreign and domestic inspections, FDA has been increasing its foreign inspections, especially at drug plants in India. India is FDA’s current top priority, as it has the largest number of drug manufacturers and is a major...
