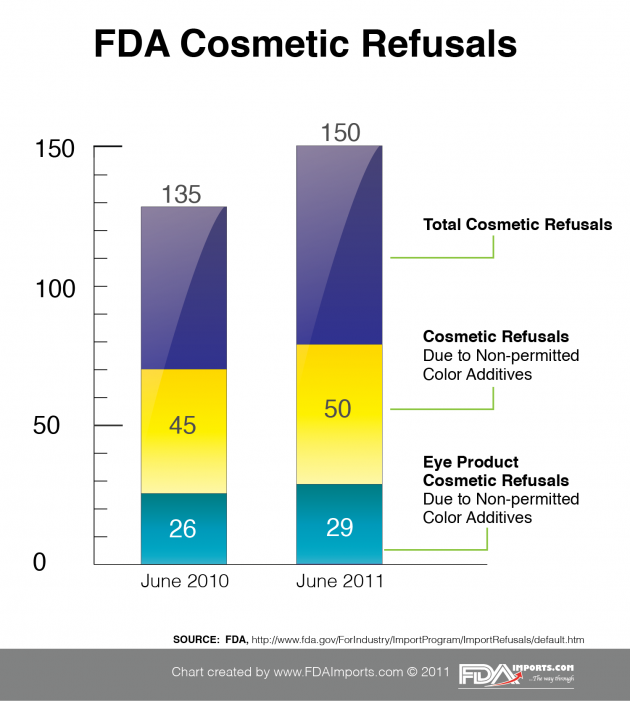
Are you a cosmetics manufacturer or importer bringing in product to the United States? The FDA is serious about regulating cosmetics, especially when it comes to color additives (see chart). Make sure you’re not making one of these common mistakes when it comes to importing your cosmetic products into the USA. The four most common mistakes are:
1. Using a color additive that is not FDA-Approved. Many manufacturers use a color additive that is approved in one country, but is not FDA-approved. For example, in Europe a cosmetic not applied on mucous membranes can include CI 11680 (Pigment Yellow 1). However in the United States, a cosmetic cannot include CI 11680 at all.
2. Using approved color additives but in the wrong product categories. The second most common mistake is when the cosmetic includes an FDA-approved color additive, but that color additive is not approved for that specific product category. For example, D&C Red No.6 is approved for general use (including lips), but not for use around the eyes. Therefore, a lipstick may include Red 6, but an eye shadow cannot.
3. Using the wrong names for the right color additives. The third most common mistake of importing cosmetics occurs when the cosmetic’s label declares the color additive according to a name other than the FDA-established name. For example, the label only states the “CI” number, instead of the name FDA has established for the color.
4. Messing up the “May Contain” statement. The last most common mistake occurs when cosmetics include non-color additives or unapproved color additives in the “May Contain” statement on the label. This is a common method of identifying all kinds of cosmetic ingredients in other countries, but it is carefully regulated in the US. FDA assumes everything added to a “May Contain” statement is a color additive, which often results in expensive FDA import detentions.
Foreign companies take note: Cosmetics, labeling and color additives are a big deal to FDA! Making any of the above mistakes can cost the importer or manufacturer some or all of the following:
– Refusal of product at U.S. port
– Destruction of entire shipment of product
– Addition of manufacturer onto Import Alert
– Future automatic detentions of cosmetic shipments
– Relabeling entire product line
– Supply chain delays, wasted time and wasted money
– Strained business relationships
A foreign cosmetic manufacturer or US importer can avoid FDA detention, FDA refusal, and possible inclusion on an FDA Import Alert list by investing in simple regulatory compliance work at the beginning. A manufacturer or importer should have its cosmetic formulations and labeling reviewed to ensure it only uses permissible color additives as well as review the label for compliance by ensuring the ingredients are named properly. These simple, but critical steps, can save manufacturers and importer from untold headaches and thousands of wasted dollars down the road.