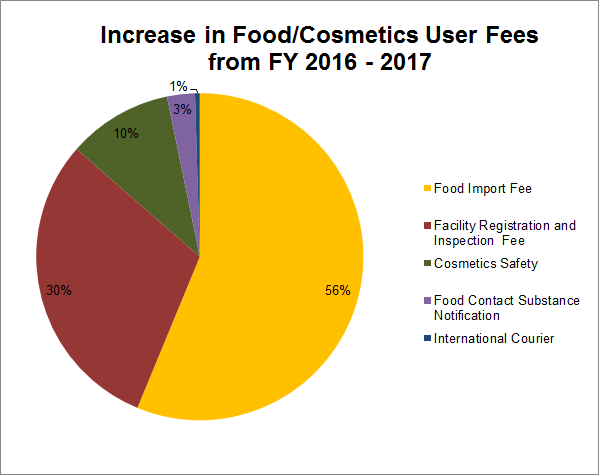
Since FY 2015, Congress has continuously raised the issue of inadequate border screening of food. Particularly, Congress has been concerned with designing an import control strategy that incorporates a comprehensive system of safeguard reviews, rather than solely relying on individual FDA reviews at the port of entry. With its proposed fiscal year (FY) budget for 2017, FDA requested an $182,464,000 overall user fee budget for Foods and Cosmetics. This is a significant increase over the enacted budget for 2016, in which FDA requested $11,586,000. The overall breakdown for the increase in user fees for Foods/Cosmetics from 2016 – 2017 is as follows:
- Proposed Food Import User Fee = +$96.1 million
The revenue from the increased proposed food import fee is intended to improve streamlining of the current import screening process – including better integration with U.S. Customs and Border Protection and increased border staff.
- Proposed Food Facility Registration/Inspection User Fee = +$51.6 million
The increase in user fee revenue for facility registration and inspections is intended to provide resources for training of FDA inspectors and compliance staff to improve targeting and risk-based efficiency of inspections.
- Cosmetics Safety = +$17.7 million
With regards to cosmetic safety, FDA intends to use the increase in user fee funds to establish a Mandatory Cosmetic Registration Program that will require domestic and foreign cosmetic labelers to register their establishments with FDA. The idea here is that the user fees will enable FDA to apply risk-based approaches to post-market monitoring of domestic and imported products, inspection, and other enforcement activities.
- Proposed Food Contact Substance Notification User Fee = +$4.7 million
The resources funded by the increase in user fees here would allow FDA to expand the Food Contact Notification Program and continue development and updates of industry guidance.
- International Courier = +$0.8 million
The increase in user fees here is intended to assist FDA staff with tracking the increased shipments of food commodities through express courier facilities – including allocating resources to conduct entry reviews, sample collections and physical exams, as well as initiate compliance actions to prevent release of unsafe products into U.S. commerce.
Figure 1: Breakdown of increase in user budget for Food/Cosmetic User Fees from FY 2016 – 2017
FDA’s Other User Fees
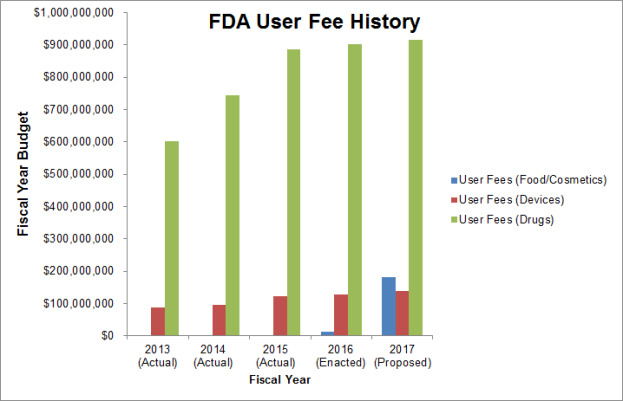
User fees for Drugs and Devices were introduced in 2013 and have increased since then; however, until recently, there were no user fee requirements for Foods (see Figure 2). Given the Foods Program’s increase in the user fee budget, it is not difficult to imagine that FDA is attempting to implement the same pay-per-use system that is used for drugs and devices. With regards to drugs and device regulation, one caveat of user fees is to establish a sort of “fee/reward” system, which actually drives profit margins for the industry as a whole. For example, a small pharmaceutical company might pay a user fee for fast track approval. The company would receive a faster-than-normal review (i.e., a reward for paying a fee).
However, this same fee/reward system may not translate as well to the Food industry as it does for the Drug and Device industries. In fact, user fee requirements may be a burden on Food companies, especially if there is no reward to be gained for paying fees. One interesting point to note is how the increase in food user fees will affect small businesses in the industry. Given FDA’s user fee history for devices and drugs, perhaps FDA will make an effort to reduce the burden on small businesses by providing fee waivers or exemptions.
Upcoming Biennial Food Facility Registrations
As of now, FDA’s budget for FY 2017 (which starts on October 1, 2016) has not been approved. Therefore, FDA’s biennial requirement that food facilities renew their registrations with FDA during the period beginning on October 1, 2016 and ending on December 31, 2016, is unchanged – at least for now. FDAImports.com will keep you informed about possible changes. If you have questions about your upcoming facility registration/renewal, please contact us.
Figure 2: FDA User Fee Budget History for Foods/Cosmetics, Drugs, and Devices